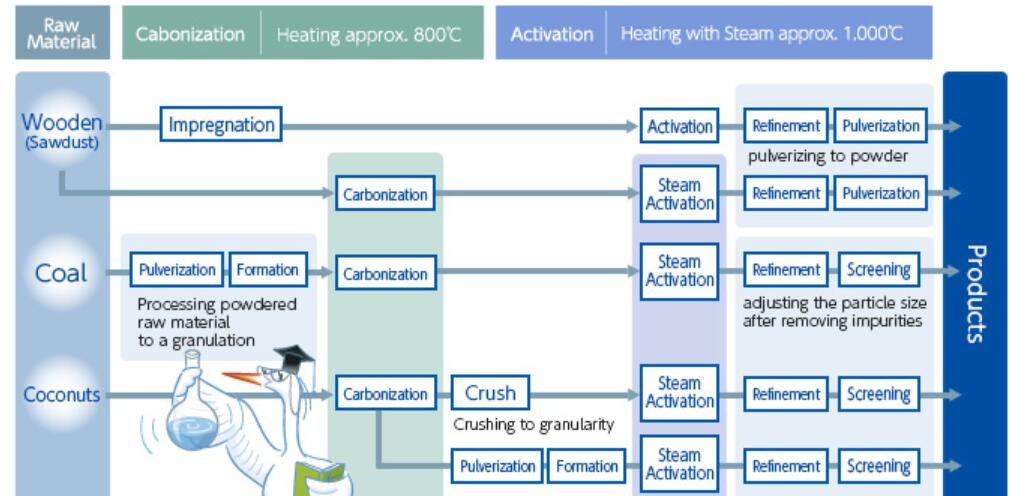
The procedure for processing activated carbon typically consists of a carbonization followed by an activation of carbonaceous material from vegetable origin. Carbonization is a heat treatment at 400-800°C which converts raw materials to carbon by minimizing the content of volatile matter and increasing the carbon content of the material. This increases the materials strength and creates an initial porous structure which is necessary if the carbon is to be activated. Adjusting the conditions of carbonization can affect the final product significantly. An increased carbonization temperature increases reactivity, but at the same time decreases the volume of pores present. This decreased volume of pores is due to an increase in the condensation of the material at higher temperatures of carbonization which yields an increase in mechanical strength. Therefore, it becomes important to choose the correct process temperature based on the desired product of carbonization.
These oxides diffuse out of the carbon resulting in a partial gasification which opens pores that were previous closed and further develops the carbons internal porous structure. In chemical activation, the carbon is reacted at high temperatures with a dehydrating agent that eliminates the majority of hydrogen and oxygen from the carbon structure. Chemical activation often combines the carbonization and activation step, but these two steps may still occur separately depending on the process. High surface areas in excess of 3,000 m2 /g have been found when using KOH as a chemical activating agent.
Activated Carbon from Different Raw Materials.
In addition to being an adsorbent used for many different purposes, activated carbon can be produced from a wealth of different raw materials, making it an incredibly versatile product that can be produced in many different areas depending on what raw material is available. Some of these materials include shells of plants, the stones of fruits, woody materials, asphalt, metal carbides, carbon blacks, scrap waste deposits from sewage, and polymer scraps. Different types of coal, which already exist in a 5 carbonaceous form with a developed pore structure, can be further processed to create activated carbon. Although activated carbon can be produced from almost any raw material, it is most cost effective and environmentally conscious to produce activated carbon from waste materials. Activated carbons produced from coconut shells have been shown to have high volumes of micropores, making them the most commonly used raw material for applications where high adsorption capacity is needed. Sawdust and other woody scrap materials also contain strongly developed microporous structures which are good for adsorption from the gas phase. Producing activated carbon from olive, plum, apricot, and peach stones yields highly homogenous adsorbents with significant hardness, resistance to abrasion and high micropore volume. PVC scrap can be activated if HCl is removed beforehand, and results in an activated carbon which is a good adsorbent for methylene blue. Activated carbons have even been produced from tire scrap. In order to distinguish between the wide range of possible precursors, it becomes necessary to evaluate the resulting physical properties after activation. When choosing a precursor the following properties are of importance: specific surface area of the pores, pore volume and pore volume distribution, composition and size of granules, and chemical structure/character of the carbon surface.
Choosing the correct precursor for the right application is very important because variation of precursor materials allows for controlling the carbons pore structure. Different precursors contain varying amounts of macropores (> 50 nm,) which 6 determine their reactivity. These macropores are not effective for adsorption, but their presence allows more channels for creation of micropores during activation. Additionally, the macropores provide more paths for adsorbate molecules to reach the micropores during adsorption.
Post time: Apr-01-2022