Activated carbon (AC) refers to the highly carbonaceous materials having high porosity and sorption ability produced from the wood, coconut shells, coal, and cones, etc. AC is one of the frequently used adsorbents utilized in various industries for the removal of numerous pollutants from water and air bodies. Since, AC synthesized from the agricultural and waste products, it has proved to be a great alternative to the traditionally used nonrenewable and expensive sources. For the preparation of AC, two basic processes, carbonization and activation, are used. In the first process, precursors are subjected to high temperatures, between 400 and 850°C, to expel out all the volatile components. High elevated temperature removes all the noncarbon components from the precursor such as hydrogen, oxygen, and nitrogen in the form of gases and tars. This process produces char having high-carbon content but low surface area and porosity. However, the second step involves the activation of previously synthesized char. Pore size enhancement during the activation process can be categorized into three: opening of previously inaccessible pores, new pore development by selective activation, and widening of existing pores.
Usually, two approaches, physical and chemical, are used for activation to get desired surface area and porosity. Physical activation involves the activation of carbonized char using oxidizing gases such as air, carbon dioxide, and steam at high temperatures (between 650 and 900°C). Carbon dioxide is usually preferred because of its pure nature, easy handling, and controllable activation process around 800°C. High pore uniformity can be obtained with carbon dioxide activation in comparison to steam. However, for physical activation, steam is much preferred as compared to carbon dioxide since AC with relatively high surface area can be produced. Due to the smaller molecule size of water, its diffusion within char’s structure occurs efficiently. Activation by steam has been found to be around two to three times higher than carbon dioxide with same degree of conversion.
However, chemical approach involves the mixing of precursor with activating agents (NaOH, KOH, and FeCl3, etc.). These activating agents acts as oxidants as well as dehydrating agents. In this approach, carbonization and activation is carried out simultaneously at comparatively lower temperature 300-500°C as compared to the physical approach. As a result, it effects the pyrolytic decomposition and, then, results in expansion of improved porous structure and high carbon yield. Major benefits of chemical over physical approach are the low temperature requirement, high microporosity structures, large surface area, and minimized reaction completion time.
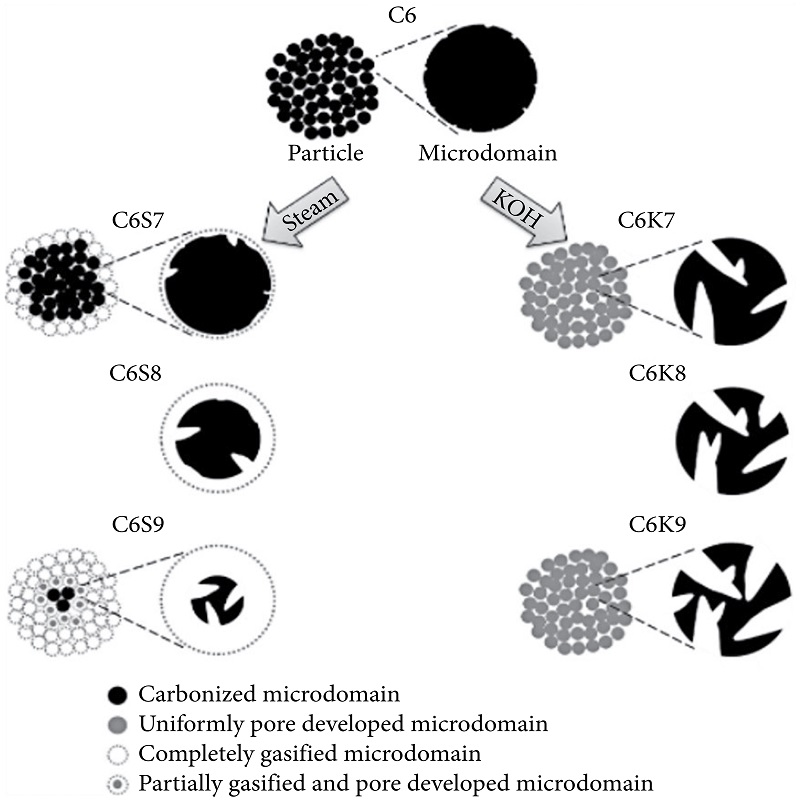
The superiority of chemical activation method can be explained on the basis of a model proposed by Kim and his coworkers [1] according to which various spherical microdomains responsible for the formation of micropores are found in the AC. On the other hand, mesopores are developed in the intermicrodomain regions. Experimentally, they formed activated carbon from phenol-based resin by chemical (using KOH) and physical (using steam) activation (Figure 1). Results showed that AC synthesized by KOH activation possessed high surface area of 2878 m2/g as compared to 2213 m2/g by steam activation. In addition, other factors such as pore size, surface area, micropore volume, and average pore width were all found to be better in KOH-activated conditions as compared to steam activated.
Differences between AC Prepared from steam activation(C6S9) and KOH activation(C6K9),respectively, explained in terms of microstructure model.
Depending upon the particle size and method of preparation, it can be categorized into three types: powered AC, granular AC, and bead AC. Powered AC is formed from fine granules having size 1 mm with average diameter range of 0.15-0.25 mm. Granular AC has comparatively larger size and less external surface area. Granular AC are used for various liquid phase and gaseous phase applications depending upon their dimension ratios. Third class: bead AC is generally synthesized from the petroleum pitch with diameter ranging from 0.35 to 0.8 mm. It is known for its high mechanical strength and low dust content. It is extensively utilized in fluidized bed applications such as water filtration due to its spherical structure.
Post time: Jun-18-2022

